ICASA aims to contribute to a substantial decrease in the proportion of ADHD patients developing an addiction/substance use disorder (SUD) and to substantially improve the detection, diagnosis and treatment of patients having both ADHD and SUD.
Research
International Survey
Treatment of comorbid ADHD and addiction problems in youth is challenging and may differ across countries and treatment systems. Since we have no international data on this topic, ICASA has taken the initiative for this survey. We are looking for national key resource persons to participate in an international survey on treatment for youths with ADHD and addiction problems across different countries in the world. The main objective of this survey is to obtain information about the organization and clinical practices related to the treatment of comorbid ADHD and addiction problems in youth (12-20 years) in different states and countries. Study outcomes will be reported in a scientific publication and will be used as a preparation for a collaborative international research project.For participation in this survey we are currently looking for national experts who have at least 2 years of work experience in national mental health policy and/or clinical experience with treating youths with addiction and/or mental health problems.
For more information you can contact: dr. Renske Spijkerman or Marrith de Jong via ICASAsurvey@parnassiagroep.nl.
INCAS
The International Naturalistic Cohort Study of ADHD and Substance Use Disorders (INCAS) is a naturalistic multicentre observational cohort study. The aim is to identify and describe clinical characteristics, treatment modalities, clinical outcome, investigate the safety profile and of pharmacological treatment in treatment seeking adult patients with SUD and ADHD. So far, 12 sites in nine countries participate in the study. The goal is to recruit 600 patients and collect information at baseline (treatment initiation), at four weeks, at three months, and at nine months after inclusion in the study.Below you can find recent information on the inclusion of subjects per site.
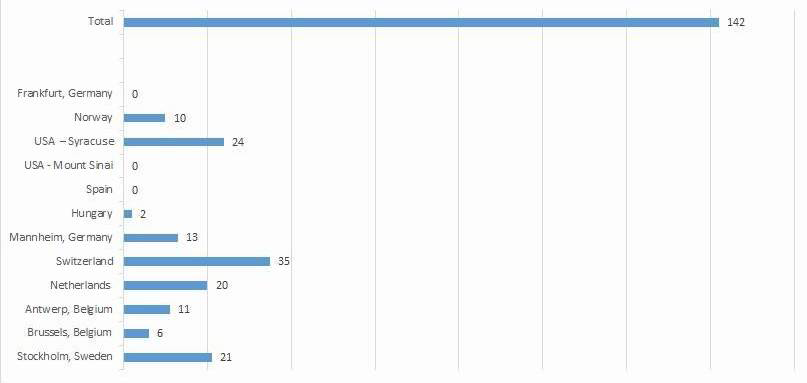
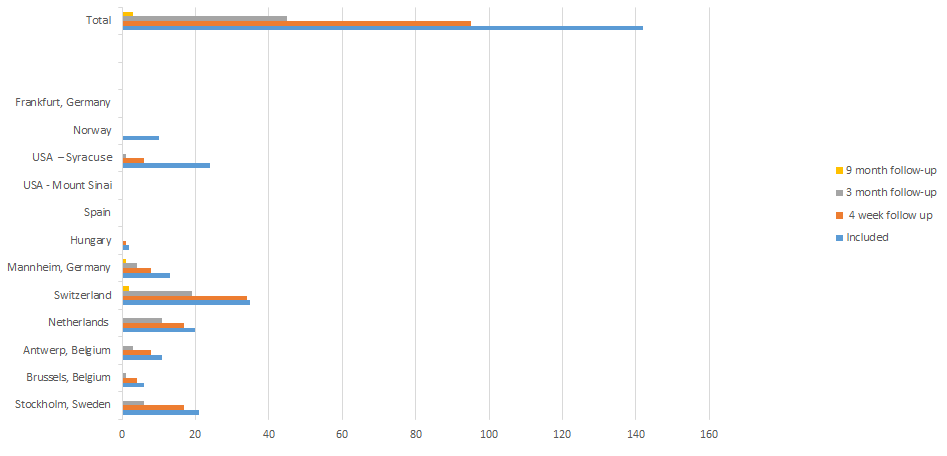
For more information on the INCAS project, please contact Christoffer Brynte or Geurt van de Glind.
IASP-2
In January 2015 a replication study of the IASP started its data collection: IASP 2. Researchers from Puerto Rico sampled data up until the end of 2017. Researchers from South Africa will continue data sampling until the end of 2018. They not only replicate the IASP study, but will add data on the development (age of onset) of ADHD, ODD and CD symptoms, and they will collect more (compared to the IASP-1 study) data on nicotine use and dependence in the groups of treatment seeking SUD patients with and without ADHD. For the latter, we will merge data with data collected in the CASP study.In the summer of 2018 we will work on several publications based on the data sampled in Puerto Rico and in the CASP project. We intend to submit these in the autumn of 2018.
CASP
The Continuous performance test for ADHD in SUD Patients study (CASP) study was developed to find out to what extend the MOXO-test could discriminate between patients with SUD only and with SUD + comorbid ADHD. The MOXO Test is a new Continuous Performance Test (CPT) and was tested in patients of addiction treatment centres in Hungary and Australia. For clinical data, the research instruments of the IASP-2 study were used.The MOXO test is accessible via the internet. The test is designed to accurately measure and describe ADHD symptoms, and differentiate between ADHD subtypes.
In this project, we tested the psychometric features of this test in patients participating in the IASP study. We will include and compare 3 groups: ADHD + SUD, SUD only, controls.
Time frame: Data sampling: August 2012 – September 2014. Due to several circumstances analyses of the data suffered delay. We work on analyzing the data in the spring of 2018 and intend to submit a publication with the results in the summer of 2018.
Funding of the CASP
Limited funding is available via Neurotech Ltd., Israel, the company who developed and owns the CPT tool. In the contracts between Neurotech and ICASA researchers it is stated that researchers will be unrestricted in their publication of results, even if these are not in favour of the accuracy of the MOXO test.
Past research projects
IASP
The IASP study (International ADHD in Substance use disorders Prevalence) was the first international multi-site study of the ICASA Foundation. Since 2013 we published important results based on the IASP data. You will find the list of IASP publications here.We included treatment seeking Substance Use Disorders patients in eight European countries: Norway, Sweden, the Netherlands, Belgium, France, Switzerland, Hungary, Spain and from two non-European countries: Australia and the United States of America. We included over 3500 SUD patients and screened them for ADHD. Over 1200 of these subjects were included in the second phase of the study, in which we did thorough diagnoses on ADHD (in both ADHD screen positives and screen negatives of the first phase of the study) and diagnoses of other comorbid mental disorders (Depression, Antisocial Personality Disorder, Borderline Personality Disorder, Bipolar Disorder).
The study investigated:
- Prevalence rates of ADHD in treatment seeking patients with Substance Use Disorders. The differences and similarities between countries and between different substances and treatment settings are being investigated
- Comorbidity in patients with and without ADHD
- The validity of screening and diagnostic procedures for ADHD in this population
- Psychometrics of the MINI-Plus ADHD module in a sample of SUD patients
- Antisocial Personality in Substance Use Disorders patients with and without ADHD
Funding of the IASP study
For an international study of this scale, central organization is crucial. Purchase of research instruments, translation of these instruments and training of the local project teams is necessary. Also, the local sites need funding for sampling the DATA. In the papers we published on the results of the IASP project, we informed the journals and readers of the sources of funding. Note that the obtained funding from pharmaceutical companies was in the period before the ICASA Foundation became a formal foundation (so before 2010).
Funding of the IASP study was split in three different parts: Part 1: Central Organization before starting up the study. The central organization was located at the Trimbos-institute in the Netherlands. Funding was obtained via participating institutes, such as the University Hospital Vall d'Hebron in Barcelona, the University of Amsterdam and the Karolinska institute in Stockholm. Furthermore, 4 pharmaceutical companies (Janssen Cilag, Eli Lilly & Company, Shire, UCB) supported this part of the study with unrestricted grants, meaning that these companies had no influence on the research methods, researchers or results. Part 2: Funding for the local participating sites. Each participating site was responsible for funding its own part of the study. Part 3: Data collection, analyses and publication of results. ICASA established a PhD position at the University of Amsterdam for doing this part of the study, which resulted in a PhD thesis in 2013. ICASA succeeded in finding funding for this position via private funding foundations: the Waterloo Foundation (UK), the Noaber Foundation (the Netherlands) and the Augeo Foundation (the Netherlands).
Vision on research
Development of addiction in children, adolescents and adults with ADHD is a serious problem with severe consequences for patients, their families and society. Diagnosis and treatment of the two disorders, especially when they are present in one patient, is complicated. The ICASA research network is convinced that high quality research is a necessary route to help solving these problems. We need scientific proof of the existence of the problem, we need to be sure of sound diagnosis and proven screening and diagnostic instruments in order to prevent over or under diagnosis of ADHD. We need to find out what works and what does not. Many researchers and groups around the world, also outside of our research network, are working on these issues. By sharing our knowledge and expertise and by making research accessible for patient-groups/subjects, we can enhance the results of the projects and accelerate the process of helping to solve the core problems: development of SUD in ADHD patients, and poor diagnosis and treatment results in patients with ADHD and SUD.
Back to top